Breast implants are prosthetics that are used to alter the shape, size, and contour of a person’s breasts. They are commonly used for breast reconstruction in women after breast cancer surgery or for correction of deformities from birth. They are also used to augment the natural appearance of breasts in women seeking cosmetic enhancements.
Getting breast implants is increasingly common and patients have been seeing positive results. However, recent developments have led to a correlation between breast implants and BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma).
There has been an increased incidence of BIA-ALCL reported mainly in America, Europe, and Australia over the years and Singapore has seen its first case recently. What is this condition and how does it affect patients who have been diagnosed or who are at risk of being exposed? Here are facts and concerns surrounding BIA-ALCL and the relation to other potential issues regarding breast implants.
What is BIA-ALCL?

In essence, BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma) is a type of lymphoma that can develop in the scar tissue and fluid around breast implants. Despite the concerns surrounding this condition, BIA-ALCL is quite rare and treatable if detected early. At this time, most of the data suggest that it occurs frequently in patients with implants with textured surfaces rather than smooth surfaces. [1]
According to HSA, there are approximately 800 incidents of BIA-ALCL globally, consisting of both confirmed and unconfirmed cases. Within Singapore, there has been one confirmed case of BIA-ALCL as of May 2019, and the patient has reported to be recuperating from the condition. [2]
BIA-ALCL is not breast cancer

BIA-ALCL is not considered a cancer of the breasts. Whilst BIA-ALCL occurs around the breast implant, it is not a breast cancer and it is classified as a lymphoma at all stages and presentation. BIA-ALCL is actually a cancer of the immune system. Clinical studies have shown that BIA-ALCL can develop in patients who have undergone breast cancer reconstruction, as well as in patients who have undergone cosmetic augmentation. [3], [4]
How does BIA-ALCL differ from lymphoma?
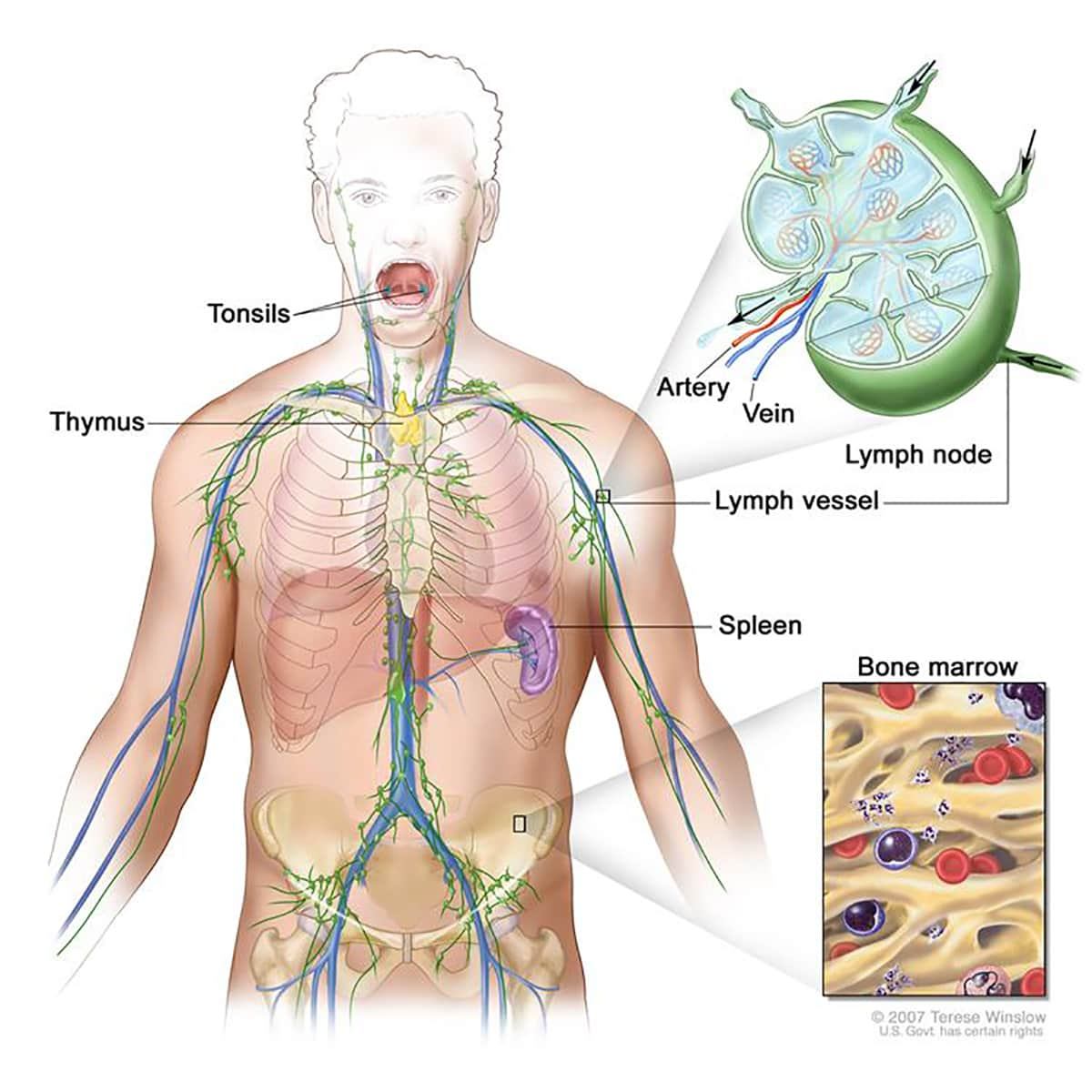
Source: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/lymphatic-system
Anaplastic Large Cell Lymphoma (ALCL) is actually a type of non-Hodgkin's lymphoma, described as a cancer of the cells in the immune system.
Lymphoma generally begins in the infection-fighting cells (called lymphocytes) found in the lymph nodes, spleen, thymus, bone marrow and other parts of the body. Specifically, BIA-ALCL develops in the scar tissue (capsule) and fluid found around the implant following breast implants. In most cases, it is localised to the capsule, but in some cases, it can spread throughout the body. [5]
What causes BIA-ALCL?

The smooth implant on the left is distinctly different from the textured implant on the right which has a rough surface.
The cause of BIA ALCL is not fully understood.
Primarily, BIA-ALCL has been associated with breast implants that have a certain texture - particularly textured implants (which have rougher surfaces that can be compared to sandpaper, unlike smooth-surfaced implants). The source of BIA-ALCL development lies within the fibrous scar capsule and in the fluid surrounding the implant and not in the breast tissue itself.
To date, there have been no confirmed cases of a BIA-ALCL in patients who only had smooth textured implants. Implant shell texturing was a modification done to reduce capsular contracture (see below). However, now it seems that this texturing process may have a link to BIA-ALCL.
Related: Can patients feel if they have capsular contracture?
There are a few theories to the development of BIA-ALCL. One of them is the biofilm theory which proposes that contamination with bacteria during surgery may lead to a biofilm that causes chronic infection and ALCL. The other theory is that the macro-texture of the silicone shell causes the body to react and triggers the immune system to produce ALCL. Yet another is the immunology response of the body to the silicone particulate material that leads to ALCL. All share chronic inflammation as a common mechanism. [6]
Global reports have outlined the prevalence of BIA-ALCL incidents with the use of ‘macro-textured’ breast implants. These ‘macro-textured’ breast implants are also associated with the highest risk of BIA-ALCL. For this reason, the HSA has recently disallowed the Allergan Natrelle™ Gel-filled Breast Implants since April 2019, which was the only registered brand of ‘macro-textured’ breast implants in Singapore. [7] [8]
What are textured breast implants?

Textured breast implants have a rough surface comparable to sandpaper. The texturing is meant to prevent them from moving around or rotating within the implant pocket created by the surgeon. This is especially important for tear-drop shaped breast implants because any movement of the breast implant can potentially be detrimental to the appearance of the patient’s breast.
The U.S. Food and Drug Administration (FDA) announced on May 02, 2019, that it has decided not to ban textured breast implants:
In 2018, textured breast implants represented less than 10% of breast implants sold in the U.S. The type of macro-textured implants targeted by some of our international counterparts represents less than 5% of breast implants sold here. At this time, the FDA does not believe that, on the basis of all available data and information, the device meets the banning standard set forth in the Federal Food, Drug and Cosmetic Act.
The FDA believes regulatory action must be based on scientific data. While the majority of women who develop BIA-ALCL have had textured implants, there are known cases in women with smooth-surface breast implants and many reports do not include the surface texture of the implant at the time of diagnosis.” - FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D., and Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health
However, other countries than the US have taken action to halt the sale of textured breast implants due to “potential risks associated with the devices, [that] outweigh the potential benefits”, including Canada, France, and Singapore. [9]
What are the symptoms of BIA-ALCL?

The most common presentation of BIA-ALCL is a large spontaneous collection of fluid (seroma) around the implant occurring at least 1 year, and on average 7-10 years after implant insertion. [4] Patients may be misdiagnosed or think they have an implant rupture. As a general rule, implant ruptures do not increase the size of the breast. Every implant will likely have a small amount of surrounding fluid (5-10ml) and this does not require biopsy or investigations. Other symptoms include masses or localised swellings and pain over the breast implant.
Unfortunately, these symptoms can be non-specific and can occur long after the implant procedure is complete and after patients have healed (sometimes even years after the initial placement). Hence we recommend patients to perform regular breast self-examination and to follow-up with their plastic surgeon after breast implants. [10]
How is BIA ALCL diagnosed?
Patients with breast implants should have a yearly review with their plastic surgeon and also keep current with their mammogram and ultrasound screening for breast cancers. Upon discovering symptoms suggestive of BIA-ALCL, patients should first consult their plastic surgeon and undergo a general physical examination. This should be followed by an ultrasound or magnetic resonance imaging (MRI) of the suspected breast. Tests may be conducted to evaluate the fluid or possible lumps around the implant and in the lymph nodes. Through this process, BIA-ALCL may be identified. Treatment can begin its course soon after. [11]
Do implants increase the risk of breast cancer?

Breast implants do not increase the risk of breast cancer. The FDA believes that women with breast implants that have textured surfaces have a very low but increased risk of developing BIA-ALCL, which is not breast cancer. ALCL may occur in breast tissue but it is a type of cancer that ultimately targets the cells of the immune system. [12]
How is BIA-ALCL treated?
Patients with a sudden fluid build-up around the implant more than a year after implant procedures should be evaluated for BIA-ALCL. Essential to the treatment of BIA-ALCL is a timely diagnosis and complete surgical excision. The surgical management involves the removal of the implant and complete removal of the capsule (total capsulectomy and complete removal of any disease or mass found in or around the concerned area of ALCL development) by experienced surgeons.
The fluid around the capsule and the tissue from the capsule will be sent for testing. The histological examination of the fluid and the capsule will confirm the diagnosis of BIA-ALCL and it will also establish the depth of the invasion and the severity of the condition. [13]
HSA has stated that the success rate for surgical treatments are increased substantially if a diagnosis is reached quickly. Early management can be the key to a seamless and effective recovery. In instances where the diagnosis is made too late and the cancer has spread to other parts of the body, patients may need to undergo further treatment including radiation, chemotherapy and targeted immunotherapy as preventive measures against continued growth of cancer.
Here is some advice from HSA (More information can be found on www.hsa.gov.sg/bia-alcl), that patients with breast implants should take note of :
- Perform regular breast self-examination.
- Follow your doctor’s instructions for routine mammography screening. Be sure to inform the facility that you have breast implants as more time may be required. Other tests such as ultrasound and MRI scans may also be recommended.
- Continue with periodic post-operation follow-ups.
- Consult your plastic surgeon if there is swelling, lumps or pain around your implant.
- Consult your plastic surgeon if you have any concerns regarding your implants and discuss the options available regarding your concerns. [2]
How do I know if a breast implant is safe?
In Singapore, breast implants are evaluated thoroughly before being allowed in the market. Evaluations for safety, quality, and efficacy are considered before use by the general public.
As mentioned, the data currently suggests that BIA-ALCL is largely associated with textured-surface breast implants, which account only for a proportion of implants. If patients have concerns, they should ask their doctors whether the implants they have used or will be using are of this textured-surface.
Whilst our knowledge about BIA-ALCL cause is derived from very low-evidence studies, there is currently no case of BIA-ALCL in any patient with a history of only smooth-textured implants. In view of these risks, I would recommend patients to consider either fat grafting or choosing a smooth implant for breast augmentation.
Having said that, there is still a place for textured implants although patients have to be counseled and informed of the risks involved. A novel implant from Establishment Labs (MotivaTM) and approved by HSA for use in Singapore was designed with a new shell to reduce the risks of complications associated with older implants whilst promising to be softer to touch and more natural in shape.
However, the longest study available for this implant is a 6-year study. Motiva breast implants are classified as smooth implants.
Nevertheless, patients should consult their doctors on the best way forward with regards to the safest options available to them. [14]
Related: The Different Types and Costs of Breast Implants
Is it hard to detect breast cancer with implants?
**
**Source
In Singapore, the Health Promotion Board encourages breast self-examination for all women above 30 and mammography screening for women aged 50 and above. Special mammogram scans can be done for patients with breast implants for breast cancer screening.
In cases where breast implants are increasing the difficulty of detection of breast cancer, there are additional screening methods aside from mammograms that can be used. Ultrasound scans and MRI scans are other options for screening the breasts for cancer and patients should consider it if their doctors advise them as such. [15]
What should I ask my doctor about BIA-ALCL?

Patients should be proactive with their concerns about BIA-ALCL and set up appointments with their doctors about any worries they may have. There are a few questions that can help guide patients towards safer implant options.
Patients should ask if the implants recommended by their doctors are HSA-approved and what are the potential complications associated with these devices. We have recognised that a breast implant (a man-made device) adds a third factor into the equation of surgery, going beyond just the surgeon and the patient. Patients should also ask how often follow-ups are needed after the augmentation or reconstruction surgery is completed.
Singapore saw its first diagnosis of cancer associated with breast implants and since then, health authorities have been on alert about initiating preventive measures for the safety of the general public. As a result, the HSA has required that manufacturers of breast implants include cautionary statements in the package inserts of any breast implants registered in the nation.
These statements revolve around the risks of BIA-ALCL. In addition, healthcare professionals have been informed of the possible association between BIA-ALCL and textured implants. The Singapore Association of Plastic Surgeons together with the Chapter of Plastic Surgeons, Academy of Medicine, Singapore have updated their members and reminded them to share on the risks of BIA-ALCL in all patients seeking breast implant for both reconstructive and aesthetic indications. [16]
What should I do if I already have textured breast implants? Do they need to be taken out?

Patients with textured breast implants do not need to have their implants removed if there are no symptoms. Consult your doctor early if you have symptoms of swelling, lumps or pain around the implant.
Do remember to continue regular yearly follow-up and keep up to date with breast cancer screening. In addition, if you are considering breast implants, discuss the options and the pros and cons of different breast implants with your doctor.
If patients with textured breast implants have decided on breast implant removal, patients have several options to pursue after the removal:
- Retaining the Post-Removal Breast as it is.
- Replacing with a New Breast Implant. Breast Explant and re-insertion of a new implant in a new place would be the best procedure of choice.
- Patients can consider Fat Grafting which would involve liposuction and replacing the removed implant with your own fat instead of a foreign body.
Related: How does removing soft breast implantations completely, partially or not removing at all compare?
What is the difference between BIA-ALCL and capsular contracture?

Capsular contracture is notorious for being one of the most common complications following breast surgeries. It is also the most common reason for reoperation and is caused by an excessive fibrotic reaction to a foreign body (in this case, the implant itself). It's a direct response of the immune system to foreign material in the human body triggered by a rejection of sorts.
It is different from ALCL, in the sense that the immune system instigates the formation of capsules of tightly-woven collagen fibers as a response to a foreign object. Factors include bacterial contamination, rupture of the breast-implant shell, leakage of the silicone-gel filling, and blood clots.
The condition is a consequence of the immune system defending the patient's bodily integrity and health, and may even reoccur in some instances.
BIA-ALCL, on the other hand, is the gradual development of cancer that attacks the cells of the immune system. [17]
Is there a link between textured implants and capsular contracture?

Since the 2000s, texturisation of silicone-filled breast implants has been shown to reduce the incidence of capsular contracture, especially in the subglandular placement of implants. [18]
To reduce the incidence of capsular contracture, surgeons would either place the implants under the muscle (with the drawback of animation deformities) or place textured implants under the breast tissue.
This explains why various implants company started to produce implants with different degree of texturisations. However, it is the same texturing process (specifically macro-texturing) that has been suspected to have a relationship with BIA-ALCL.
What is capsular contracture and How is it treated?

When foreign material such as a silicone breast implant is placed in the body, a capsule of fibrous tissue forms around it. This capsule is initially very thin and soft (Grade I). However, with time, some capsules undergo progressive thickening and in some patients, this may lead to problems.
Also read: Are saline or silicone breast implants better?
All patients with a breast implant will have a breast capsule but not all capsules will cause problems. Capsular contracture is graded on a scale of I to IV, with I being mild and IV being the most severe and causing pain. Grade I and II capsules do not present any problems and are left alone. However severe cases of capsular contracture (Grade III or IV) can cause either distortion of the breasts or pain.
In patients who want to have the implants removed without replacement and without removal of capsules, studies have shown that the capsule will be resorbed over time. [19]
However, in patients who want to change their implants, the best solution would be the removal of the contracted capsule (partial or total capsulectomy) and replacement with a new implant in a separate pocket within the breast. This has been shown to lead to reduced contracture recurrence rates. [20]
Read more on breast explant surgery in this Q&A.
How can capsular contracture be prevented?
Bleeding can be a primary cause of contracture, especially significant bleeding around the implant shortly after the surgery (this is known as hematoma). If this bleeding is not resolved, old blood can stimulate the tightening of the scar tissue capsule which leads to the complication.
Doctors may ask patients to avoid medication and supplements for about two weeks before surgery as this may increase the risk of bleeding. Fish oil, extra vitamin E, Aspirin, Ibuprofen and other substances could trigger excessive bleeding and should be avoided.
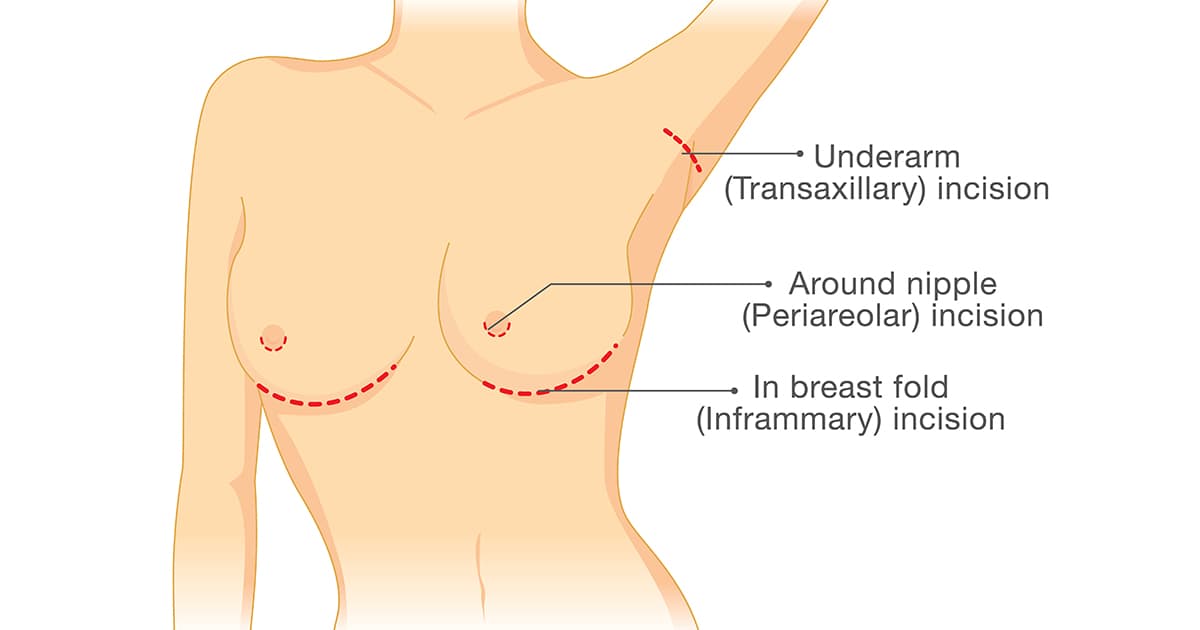
Bacteria and biofilm can also lead to an emergence of the problem even after treatment. Prevention is key. Therefore, doctors may opt for a surgical method called inframammary fold incision (which helps reduce the likelihood of bacteria accumulation during the procedure). [21]
Other ways of preventing capsular contracture include placing the breast implant under the muscle but some patients do not like that due to animation deformities.
As mentioned texturisation is another method of preventing capsular contracture, especially for implants above the muscle. A new 6th generation implant known as MotivaTM from Establishment Labs has promising early data suggesting reduced capsular contracture rates and no case of BIA-ALCL at present due to a novel implant shell surface. [22]
Ultimately, having breast implants are a personal choice that must be made by patients before they proceed with any procedure. In many cases, surgeries are not compulsory and they aren’t something that patients should rush into. Surgical procedures often come with risks, even if they are rare, and it’s important that patients do their own research and consult their plastic surgeons about the benefits and risks of opting for surgery. It’s important that patients find a doctor that they can communicate with well, as communication can be key to proper treatment and management.
Breast implants are considered safe. In fact, they are among the most-studied devices in medicine. So, as long as patients exercise caution and choose certified professionals to guide them through the process, the success rates are still significantly high.
Dr Terence Goh is a Plastic Surgeon at AZATACA Plastic Surgery and is currently the president of the Singapore Association of Plastic Surgeons, and the vice-chairman to the Chapter of Plastic Surgeons, Academy of Medicine, Singapore. He practices both cosmetic surgery and reconstructive surgery. His area of specialty is in reconstructive microsurgery including breast reconstruction, reconstruction of head and neck cancers and lower limb salvage. His cosmetic interests include rhinoplasty, eyelid surgery, breast surgery, and mummy makeovers.
Would you like to ask any related health questions?
You can Ask A Doctor right away, or view the complete list of Human Sessions.
Article medically reviewed by Dr Terence Goh